|
Structural control of polymer blendsIntroductionThe properties of materials depend strongly upon their micro- and nano-scale structures, and control of these structures is essential for more exotic applications, such as electronics and photonics. Polymer blends are particularly susceptible to forming intricate structures as the components tend to separate into distinct phases. In principle, phase separation can be used to access a wide range of interesting and potentially useful structural motifs at a range of length scales. Normally, however, phase separation in polymer blends is poorly controlled, and this is reflected in a disordered final structure. Researchers at the Polymer IRC, led by Nigel Clarke (Durham), have been tackling this problem by developing a number of strategies that allow us to define product structure using phase separation techniques. A kinetic approach to morphology controlThe kinetic approach relies on fine control of the processing temperature and time for the polymer mixture. Particles of one polymer (A) are dispersed in a matrix of a second polymer (B). At a suitable temperature the interface between the particle and matrix broadens, as A slowly dissolves in B. If the polymer is quenched prior to complete dissolution, however, a phase separated structure results, which can be modelled using Cahn-Hilliard theory. The movie (right) shows a model study of the dissolution and quench process. The movie runs from the point at which the polymer mixture is quenched. The problems with this technique are that most chemically different polymers are either immiscible or only partially miscible, so interfaces can be hard to form. In the absence of an interface, the best microstructure that can be formed is a simple physical dispersion. Chemically directed phase separationMany polymer mixes will only form homogenous solutions, either in a mutual solvent, or at elevated temperatures. As the solvent evaporates, or the temperature falls, therefore, the components start to separate.
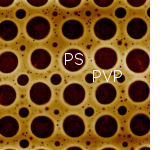
Piers Andrew and Wilhelm Huck at Cambridge University have recently found that this separation process can be directed using chemically patterned surfaces. Surface patterns can be prepared by micro contact printing, providing great scope for precise control of polymer micro-structure. The micrograph (left, by kind permission of Piers Andrew and Wilhelm Huck, Cambridge University) shows the effect of spin casting a solution of poly(2-vinylpyridine) (paler colour, indicated by 'PVP' in the micrograph) and poly(styrene) (indicated by PS in the micrograph) from THF (Tetrahydrofuran) onto a chemically patterned hydrophilic grid, overlaying a hydrophobic ground. The micrograph has a side of approximately 20µm. In this chemically directed separation, the poly(styrene) separates to form droplets over the hydrophilic regions. These droplets are not in equilibrium, but arise from the natural length scale selection process associated with spinodal decomposition. Our model of the process, shown in the movie (right), helps us to understand the origin of this unexpected behaviour. At the start of the movie (A), you can see the pattern just after phase separation is initiated. There is a ring rich in polystyrene (PS - shown in red) around the edge of the grid squares, giving circular patterns, and just outside a depletion zone/ring which is rich in PVP (blue). Shortly after (B), beads of PS have formed, giving a situation very similar to the situation shown in the micrograph, with most PS over the holes in the grid, but small droplets over the grid and larger ones over intersections of the grid lines. The situation at C shows the hypothetical end point (thermodynamic equilibrium) with all PS over hydrophobic zones, and PVP over hydrophilic grid. |
|
|
|
|
© The Polymer IRC 2004, design www.cookandkaye.co.uk /
Webmaster /
Disclaimer /
Privacy The IRC includes the University of Bradford, the University of Durham, the University of Leeds and the University of Sheffield. | |